Introduction to Hydrogen as an Energy Source

Arien Afsarpour
Battery and energy storage engineer
You’ve probably heard good and bad things about hydrogen. But whatever people might say, it’s not going away. Join Arien Afsarpour as he explores the basics of hydrogen.
You’ve probably heard good and bad things about hydrogen. But whatever people might say, it’s not going away. Join Arien Afsarpour as he explores the basics of hydrogen.
Subscribe to watch
Access this and all of the content on our platform by signing up for a 7-day free trial.

Introduction to Hydrogen as an Energy Source
10 mins 43 secs
Key learning objectives:
Understand what hydrogen is
Outline hydrogen’s benefits and drawbacks
Identify the applications of hydrogen
Overview:
In its non-naturally occurring gaseous form, hydrogen has the highest specific energy of all combustible fuels. However, due to its low density and buoyancy it presents some practical challenges, namely storage and embrittlement. Global demand for hydrogen was approximately 90 million tons in 2020, with around 95% of that amount used in petroleum refining and chemical production. Most demand is fulfilled by fossil fuel derived sources (black, grey, brown and blue hydrogen). But there is growing demand for green hydrogen, produced via water electrolysis and powered by renewable energy. Where fuel cells take hydrogen and oxygen to make electricity, electrolysers do the reverse, making hydrogen and oxygen from electricity. When it comes to the safety of hydrogen, storage is a higher concern than the gas itself. These concerns can be quelled by implementing ventilation and leak detection. The need to decarbonise is clear and the ability to produce hydrogen domestically will be essential to ensuring security in food and metal production.
Subscribe to watch
Access this and all of the content on our platform by signing up for a 7-day free trial.
What is hydrogen gas (H2)?
In its non-naturally occurring gaseous form, hydrogen exists as H2, which has the highest specific energy (expressed in kilowatt hours per kilogram) of all combustible fuels (approximately three times that of gasoline).
What are the drawbacks of hydrogen?
1. Hydrogen is the lightest of all gases, making it extremely buoyant and diffusive. This presents two main challenges, namely poor energy density and difficulty to compress and store.
2. Hydrogen is able to insert itself into the crystalline structures of metals commonly used for storage vessels and pipework, leading to hydrogen embrittlement which causes degradation in the mechanical strength of these metals.
Between embrittlement and its propensity to leak, the latter presents the more practical day-to-day challenge in designing robust hydrogen-fuelled systems.
What are the practical applications for hydrogen?
Hydrogen is already a significant industrial chemical, consumed by the oil and gas industry in processes to remove sulphur from natural gas and refined petroleum products, in the production of ammonia for fertilisers and refrigeration, for refining metals, and the production of hydrogenated vegetable oils for human consumption among many others.
What is the current global demand for hydrogen?
Global demand for hydrogen was approximately 90 million tons in 2020, with around 95% of that amount used in petroleum refining and chemical production. Nearly all of that demand was filled by fossil fuel-derived hydrogen, and was responsible for the emission of 900 million tons of carbon dioxide.
What is the hydrogen colour spectrum?
- Black hydrogen. The use of black coal to produce hydrogen.
- Brown hydrogen. The use of lignite (brown coal) to produce hydrogen.
- Blue hydrogen. The use of natural gas and heated water to produce hydrogen. This method uses carbon capture and storage (CCS) to store the carbon produced.
- Grey hydrogen. The use of natural gas and heated water to produce hydrogen. Unlike blue hydrogen, this method does not store the carbon produced.
- Green hydrogen. The use of renewable energy to produce hydrogen. This method does not create carbon dioxide.
- Pink hydrogen. The use of nuclear energy to produce hydrogen.
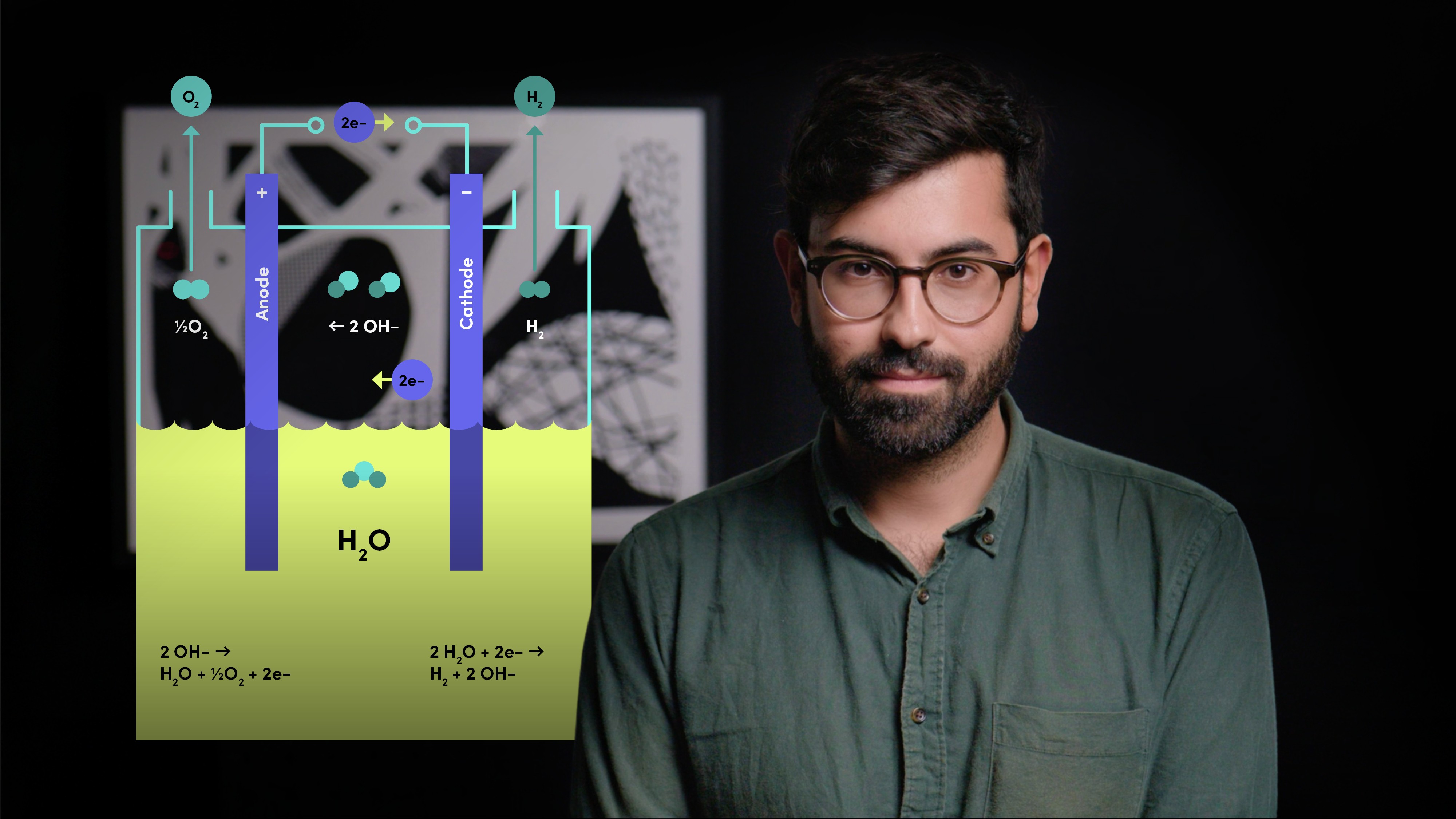
What is the difference between fuel cells and electrolysers?
Fuel cells take hydrogen and oxygen, and make electricity. Electrolysers do the reverse, making hydrogen and oxygen from electricity.
What is hydrogen’s potential as a transport fuel?
The hydrogen user experience is similar to that of petrol and diesel today. You drive up to the pump, or dispenser, open the filling door of your vehicle, and bring the nozzle up to the filler port. Although this sounds futuristic, hydrogen powered buses have been used in routes across the UK and Europe.
Road transport in the EU consumed 270 million tons of oil equivalent energy in 2019 which approximates to about 3140 terawatt hours. By comparison, the lower heating value of 90 million tons of hydrogen (the total global demand in 2020), approximates to around 3000 terawatt hours. There are potential future opportunities in developing dual-purpose hydrogen production where surpluses in supply to industrial customers could be diverted to transport.
Subscribe to watch
Access this and all of the content on our platform by signing up for a 7-day free trial.

Arien Afsarpour
There are no available Videos from "Arien Afsarpour"